Transcranial Magnetic Stimulation (TMS)
If your drug therapy doesn't work or produces intolerable side effects, TheraMind Centers have the alternative. Transcranial magnetic stimulation (TMS) is a safe and highly effective, FDA-approved, non-drug, non-invasive treatment for people suffering from depression, anxiety, and other mood conditions.

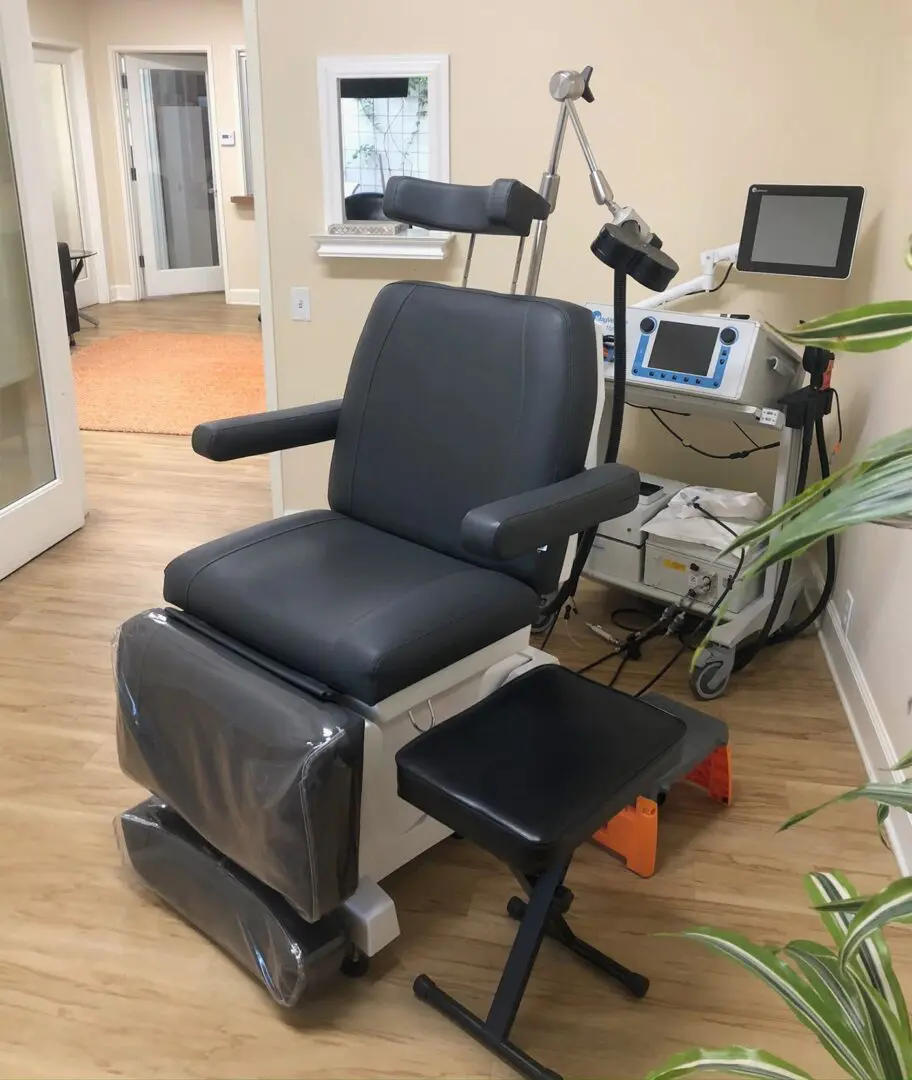
What is TMS?
TMS therapy uses magnetic pulses of different frequencies and different strengths to stimulate (or inhibit) brain activity in a localized portion of the brain. There are several FDA-approved manufacturers of TMS equipment. While there are differences among them with respect to certain engineering aspects, each is designed to provide a magnetic stimulus to neurons in the brain.
Why TMS?
TMS, transcranial magnetic stimulation, is a safe and effective, non-drug, non-invasive treatment for people suffering from depression, anxiety, and other mood and certain neurological disorders where traditional drug therapy either doesn't work or produces intolerable side effects.
Who is It for?
TMS has been safely and effectively used, among other conditions, to treat:
Our Approach
TMS is a medical procedure requiring a prescription from a licensed physician. TMS is an outpatient procedure that requires no anesthesia or sedation. For patients suffering from depression, our TMS-certified physicians screen for any contrary indications and determine if TMS is an appropriate treatment for each patient. Once the determination has been made that the patient is a good candidate for TMS therapy and a patient-specific treatment protocol has been decided upon, one of our TMS-certified doctors and a TheraMind TMS Technician perform a Motor Threshold determination (MT) on the patient, and the TMS Technician is given a set of treatment parameters by the physician for subsequent TMS sessions for that patient. All TheraMind Centers are equipped to provide repetitive TMS therapy (TMS) as well as intermittent theta burst (iTBS) TMS therapy.
A course of treatment for depression typically consists of thirty-six (36) treatments, usually five treatments a week for six weeks with three weeks of taper following, although the treatment schedule may vary based on patient response and physician prescription. A single-side treatment session normally lasts about 20 minutes for TMS and 5 minutes for iTBS, during which time patients are awake, alert, and comfortable. Our TMS Technicians are present throughout the treatment and interact with the patient while continuously monitoring the treatment to ensure the patient's comfort and the maximum efficacy of the treatment. Weekly reports of patient progress are submitted to all referring physicians and our TMS-certified medical professionals. TMS is generally considered to be free of side effects with the rare exception of headache and localized discomfort of the scalp at the treatment site, which are easily mitigated through reorientation of the magnet and/or reduction in treatment intensity.


History of TMS
In 1985, English physicist Dr. Anthony T. Barker and two other doctors first demonstrated the use of transcranial magnetic stimulation to cause twitching in the hand of a human being by stimulating the motor cortex of the brain hemisphere opposite that of the hand. This development led to an acceleration of research into and greatly enhanced understanding of how the brain works and the use of TMS to change the brain and treat diseases like depression. TMS therapy was approved by the FDA in October 2008 for the treatment of Major Depressive Disorder (MDD) and, within two years, had become so accepted by the medical community as an effective treatment for depression that the American Psychiatric Association began recommending TMS as a second-line treatment for depression. TMS has since also been approved by the FDA for OCD and Migraines.

Side Effects
TMS is generally considered to be free of side effects, with the exception of headache and localized discomfort of the scalp at the treatment site, both of which are thankfully rare and fairly easily mitigated.
References
- Jay C. Fournier, MA; Robert J. DeRubeis, Ph.D.; Steven D. Hollon, Ph.D.; et al., "Antidepressant Drug Effects and Depression, A Patient-Level Meta-Analysis," JAMA. 2010;303(1):47-53. doi:10.1001/jama.2009.1943
- "New Data Show Lack of Efficacy for Antidepressants." MadinAmerica.com, Mad in America, https://www.madinamerica.com/2017/02/new-data-showslack-efficacy-antidepressants/. February 27, 2017.
- Cole, E.J., Stimpson, K.H., Bentzley, B.S., Gulser, M., Cherian, K., Tischler, C., Nejad, R., et al. (2020 Apr 7). Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am J Psychiatry. appiajp201919070720. doi: 10.1176/appi.ajp.2019.19070720. [Online ahead of print.]
- O'Reardon, John P., et al., "Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial," Biol Psychiatry 2007; 62-1208-1216.
- Carpenter, Linda L., et al., "Transcranial Magnetic Stimulation (TMS) for Major Depression: A Multisite, Naturalistic, Observational Study of Acute Treatment Outcomes in Clinical Practice," PubMed 2012 July; 29(7): 587-96.
Other Resources
- Janicak, Philip G., et al., "Transcranial Magnetic Stimulation in the Treatment of Major Depressive Disorder: A Comprehensive Summary of Safety Experience from Acute Exposure, Extended Exposure, and During Reintroduction Treatment," J Clinical Psychiatry 69:2, February 2008.
- Kozel, Andrew F., et al., "Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: A randomized clinical trial Journal of Affective Disorders," March 15, 2018, Vol. 229, 506-514.

